-
One thing experts agree on: The damage from the funding cuts will be varied and immense.
-
A new training program teaches workers to stop the baby talk and address older people as adults.
-
The drugmaker said it would stop studying danuglipron after a participant in one of its trials experienced a possible drug-induced liver injury that ended once the person stopped the treatment.
-
A new HHS rule would require Medicare and Medicaid to cover drugs like Wegovy or Zepbound for a large segment of Americans. But it’s unclear if it will will have support of the Trump administration.
-
The state sued the FDA over what it said was a “reckless delay” in approving its drug importation plan. Nearly a year after the federal officials gave the green light, the program has yet to begin.
-
The guidelines took years to finalize, but while regulators were drafting them, a new trend emerged: online pharmaceutical influencers with little government oversight.
-
Criticism of prescription drug middlemen has intensified recently in the wake of a federal agency’s actions and legislative reform attempts.
-
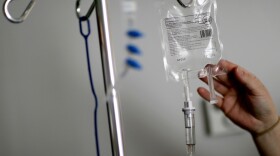
A woman ended up stranded in the hospital because CVS stopped providing the IV nutrition she needs to survive at home. Without it, she’d starve.
-
The Big Three pharmacy benefit managers say they return nearly all the rebates they get from drugmakers to the employers and insurers who hire them. But most employers seem to doubt that.
-
The pharmaceutical industry has invented a new art form: finding ways to make their wares seem like joyous must-have treatments, while often minimizing lackluster efficacy and risks.
Play Live Radio
Next Up:
0:00
0:00
Available On Air Stations