-
AstraZeneca’s drug, to be sold under the brand name Beyfortus, is a laboratory-made version of an antibody that helps the immune system fight off RSV.
-
Drug manufacturers invested in Russia’s pharmaceutical industry contend international humanitarian law requires they continue manufacturing and selling their products there, even while condemning the Ukraine invasion.
-
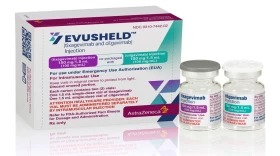
Unlike other monoclonal antibody therapies, Evusheld is not meant to treat active coronavirus infection. Instead, it's preventative medicine for people with compromised immune systems.
-
Oxford-AstraZeneca promised its COVID-19 vaccine would be effective, cheap and available worldwide. Five months after its launch, the path forward has been anything but smooth.
-
President Biden's commitment Monday to donate doses of Moderna, Pfizer and Johnson & Johnson vaccines adds to an earlier promise of 60 million doses of AstraZeneca.
-
The head of the European Union's executive arm has announced plans for a major contract extension for COVID-19 vaccines with Pfizer.
-
The European Medicines Agency placed no new restrictions on using the vaccine in people 18 and over. The agency says based on the available evidence, it was not able to identify specific risk factors.
-
Updated study results say the vaccine is 76% effective against symptomatic COVID-19 and 100% effective against severe disease. Independent monitors had been concerned about a previous report.
Play Live Radio
Next Up:
0:00
0:00
Available On Air Stations