-
The Food and Drug Administration says the Eli Lilly drug is no longer approved for emergency use because it is ineffective against current omicron strains.
-
In the past several months, new treatments have emerged, which are most effective within the first five days of symptoms but patients may have a hard time knowing whether they qualify.
-
The federal “test-to-treat” program was designed to be a one-stop shop for people to get tested and receive treatment. But as cases rise again, many communities have no participating locations, and website bugs make it difficult to book an appointment at the biggest participant.
-
The Biden administration has asked Congress to allocate $22.5 billion more for pandemic relief. But the funding is stalled and the effects are already being felt.
-
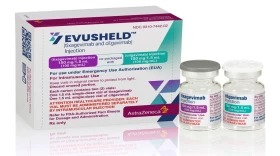
At least 7 million immunocompromised people could benefit from the monoclonal antibody injections designed to prevent COVID. The government says it has enough doses for a fraction of those in need ― and it doesn’t have the money to buy more.
-
States and health providers report they've dispensed less than half their supply from the government, raising fears that the drugs may go to waste while people who could benefit get sicker.
-
Unlike other monoclonal antibody therapies, Evusheld is not meant to treat active coronavirus infection. Instead, it's preventative medicine for people with compromised immune systems.
-
The state's 1st District Court of Appeal detailed reasons for upholding a circuit judge's decision to reject efforts by the family of a dying patient at Mayo Clinic Jacksonville.
-
Treatments are only available for patients at high risk for developing severe COVID-19. Select pharmacies and health facilities have supplies.
-
Seminole County Emergency Manager Alan Harris says some sites in the county offer sotrovimab. which remains authorized for use against omicron but is in short supply.
Play Live Radio
Next Up:
0:00
0:00
Available On Air Stations